Research
The research focuses of our lab are:
1) Light-driven production of hydrogen by a protein fusion complex of hydrogenase and photosystem I
2) Electricity production using photosystem I (PSI) from cyanobacteria when linked improve the selectivity and efficiency of electron transfer.
3) Insertion of photosystem I into lipid bilayer membranes to study the stability and electron transfer of photosystem I within an in vitro biomimetic environment
Coupled with biomolecular engineering, we also use quantitative simulation to understand physical drivers of how individual protein structures interact in fusions with other proteins or surfaces.
Project 1:
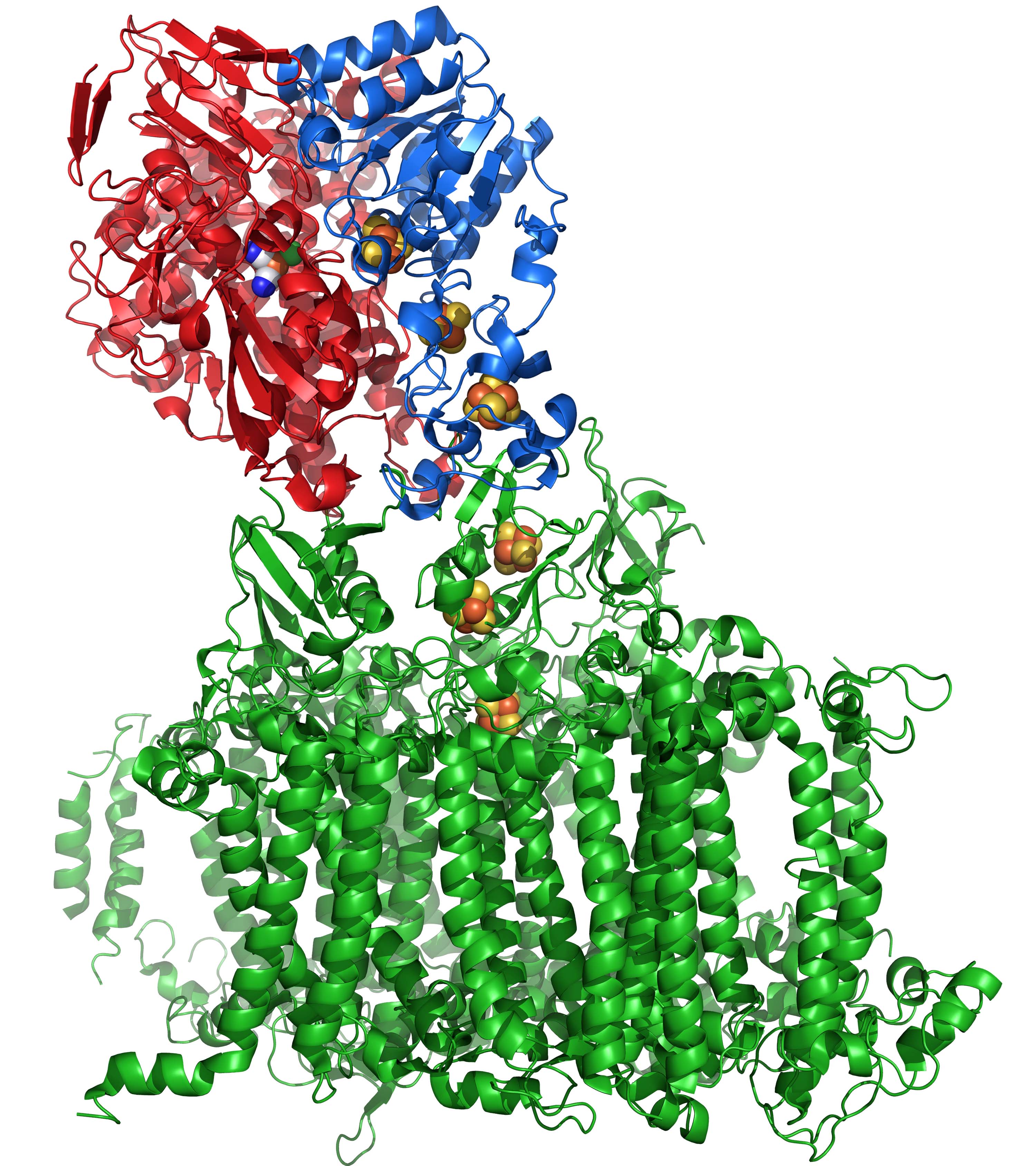
Photosystem I (PSI) accomplishes the unique biological function of converting solar energy into reducing power, and it has previously been demonstrated that aqueous suspensions of isolated PSI and colloidal platinum catalyst are capable of sustained photo-evolution of hydrogen. Another interesting and less expensive approach involves the coupling of PSI with a hydrogenase enzyme.
Hydrogenases are metalloproteins that are found in Archaea, obligate and facultative anaerobic bacteria, and some eukaryotes that catalyze reversible and selective conversion of H2 and 2H+ and are classified based on their metal content: di-iron [Fe-Fe] and nickel-iron [Ni-Fe]. The reaction of [Fe-Fe]-hydrogenases with O2 is physiologically irreversible, resulting in a damaged enzyme, whereas [Ni-Fe]-hydrogenases can be reactivated upon reduction. Several photosynthetic organisms possess native hydrogenases of the [Ni-Fe] class, and can evolve hydrogen gas under anoxic conditions.
In our lab, we work with the “O2-tolerant” hydrogenase from Ralstonia eutropha, a gram negative soil bacterium. Like other [Ni-Fe]-hydrogenases, the one from Ralstonia is comprised of two subunits: the large subunit (hoxG), which contains the active site (shown in the red subunit above), and the small subunit (hoxK), which contains the iron-sulfur clusters (within the blue subunit). The unique [4Fe-3S] structure of proximal Fe-S cluster is believed to impart rapid electron delivery to the active site upon exposure to O2, for O2 tolerance.
Unfortunately, in vivo cyanobacterial hydrogen production falls well below the theoretical maximum due to two factors: (1) the general oxygen sensitivity of hydrogenases and the specific tendency of [NiFe]-hydrogenases to favor the hydrogen uptake reaction and (2) the electron transfer from PSI to hydrogenase must compete with electron transfer to the Calvin cycle. Therefore, our intent is to focus on in vitro applications involving site-specific fusions of PSI from Synechocystis sp. PCC 6803 and the membrane-bound hydrogenase (MBH) of Ralstonia eutropha, an “oxygen-tolerant” hydrogenase which exhibits activity in the presence of O2. Using a combination of experimental and computational methods, we hope to gain new insights into electron transport in these redox proteins.
Project 2:

With a quantum efficiency of near unity and 1 V potential, the photosynthetic reaction center, photosystem I (PSI), is an ideal candidate for incorporation into a steady-state photovoltaic device. With cheap and easy extraction, compared to traditional photovoltaic materials, PSI can be adsorbed or linked to conductive or semi-conductive materials to produce electricity.
Current efforts have showed that PSI can be attached to conductive materials via self-assembled monolayers, covalently linked to gold by targeting cysteine residues of the protein complex, or deposited via vacuum or electric field assist. Though these methods are effective for depositing uniform monolayers and multilayers of PSI, their orientation is unknown. Orientation of the PSI complex is important for obtaining useful current density. PSI complexes must be oriented in the same orientation, such that different orientations do not produce competing currents that would cancel each other out.
To overcome the limited current densities in monolayers due to lack of protein orientation, we have used the highly selective sortase-mediated ligation (SML) to attach the PSI complex from Synechocystis sp. PCC 6803 in a desired orientation to enhance electron transfer to a conductive gold surface to convert solar energy into electrical energy. Sortase A is an enzyme found in Gram-positive bacteria that recognizes substrates containing an LPXTG sequence and catalyzes the cleavage of the amide bond between the threonine and the glycine, generating a thioester intermediate. The intermediate undergoes nucleophilic attack by an amino group of an amino-terminated (Gly)3-decorated gold surface. We have engineered subunits of PSI to contain a LPETG-sortase recognition sequence on the exposed C-termini of the protein that are likely to orient the complexes uniformly and in a favorable orientation for electron transfer. Photochronoamperometric measurements have been used to determine if sortase-mediated ligation can control PSI orientation and improve current density based on current production.
Project 3:
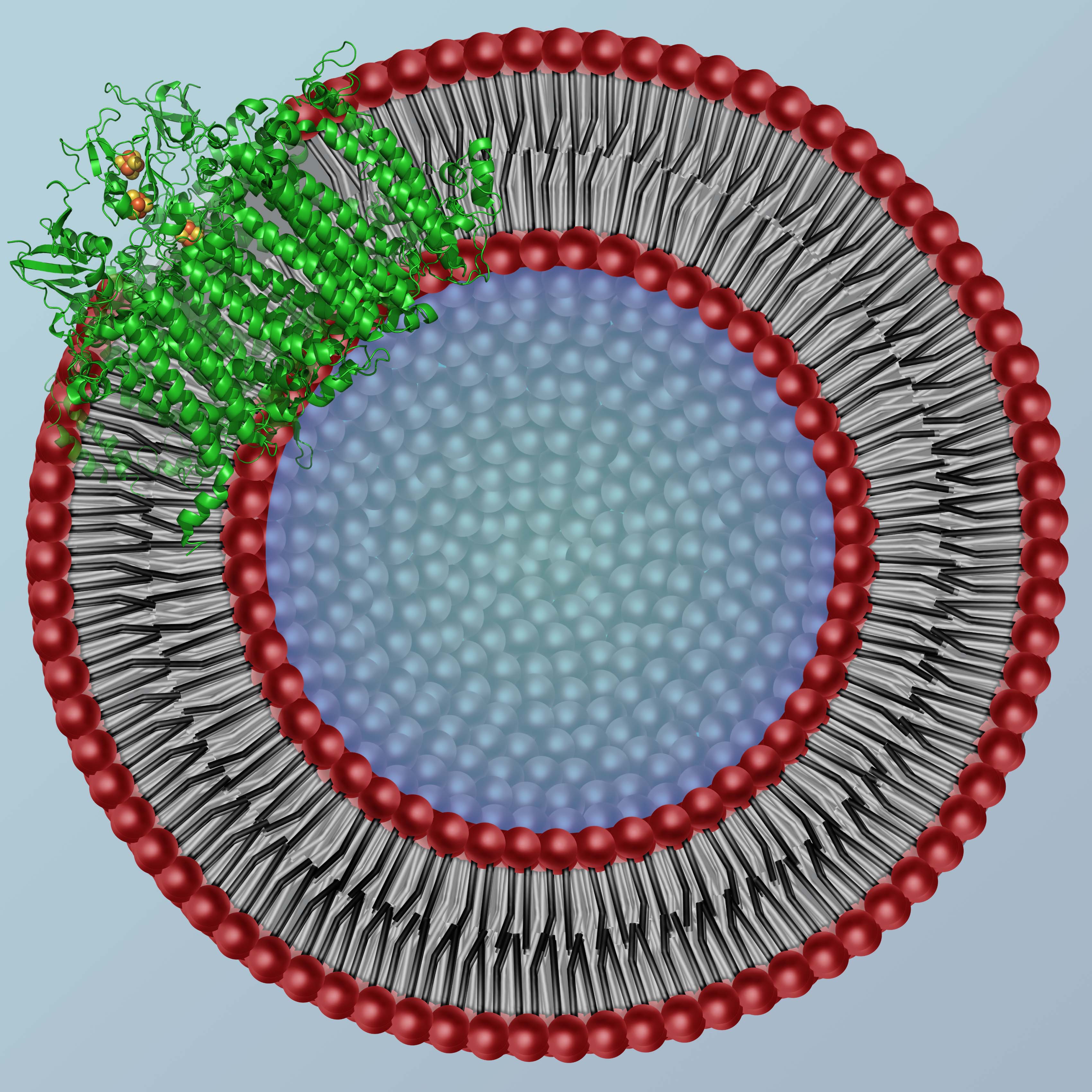
Cell structure is maintained through complex interactions of phospholipids and proteins to form cell membranes. The membranes act as protective barriers against toxins and give functionality and stability to proteins embedded in the phospholipid bilayer.
In cyanobacteria and green plants, photosynthetic complexes are amongst the proteins in the thylakoid membrane. These complexes are responsible for converting photons into usable energy. Photosystem I (PSI), part of this pathway, transfers electrons across the thylakoid membrane to form NADPH. Currently, PSI is predominantly studied in vitro while solubilized in detergent.
Recent work in our lab has shown that the detergents used to solubilize membrane proteins have a significant effect on protein structure and dynamics. A system where lipids provide the protection for the hydrophobic transmembrane domains is more appropriate for comparison to the natural system for PSI. We are currently studying the effects of membrane constituents on protein-lipid structure and dynamics by using vesicles to simulate in vivo conditions. We are interested in gaining insight into PSI activity and stability in vesicles with hope of extending this method to probe the full photosynthetic chain.
Other Projects
We have used molecular dynamics simulation to analyze the possible PSI-hydrogenase termini combinations to reduce the number of potential complexes to experimentally form and screen based on the likelihood the fusion complex would be able to transfer electrons from PSI to hydrogenase for hydrogen production. Using simulation we can analyze linkages via sortase-mediated ligation to determine distances favoring molecular wire linkages between Fe-S centers of proteins and surfaces for enhanced electron transfer.
We also use laser flash photolysis, a tool used to determine reaction kinetics for chemical systems. Using this tool, we can probe the reaction kinetics of PSI within a reduction pathway to form hydrogen from proton and electron donors.